Remote Medical International (RMI) has talked about rapid COVID-19 Testing before in the blog. This time, we’re talking specifically about the different types of rapid testing options and how they work.
Detecting the Coronavirus
To understand the different types of rapid tests available for COVID-19 testing, it is important to first understand the targets (called “biomarkersâ€) of COVID-19 infection that a test can detect in the body as well as how the levels of a particular biomarker change over the course of the illness (as shown in the figure below).
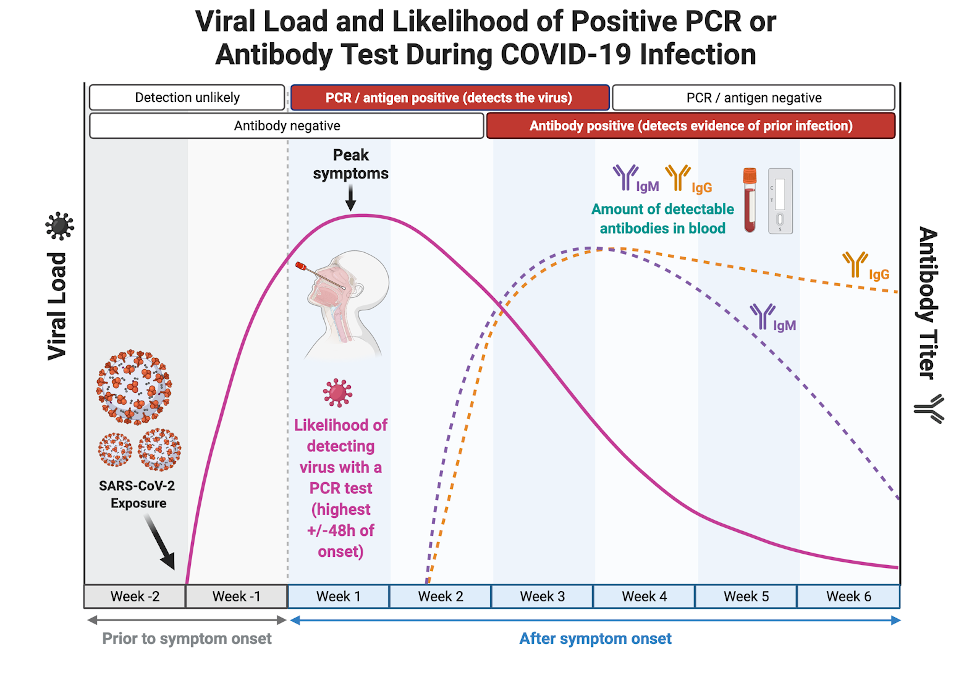
Changes in the levels of testable COVID-19 infection biomarkers over time. Although the viral particles (purple line) remain in the body during the infection, their concentration in different organ systems varies throughout the course of illness. The ability to detect viral material through oral or nasal sampling is greatest during the acute phase of the illness when the highest concentration of viral material is present in the upper respiratory tract where it can be easily collected.
Generally speaking, rapid tests can be divided into a) those that detect the presence of pieces from the actual SARS-CoV-2 (antigen tests), and b) those that detect evidence of current or past infection in cells produced by the patient’s immune system (antibody tests).
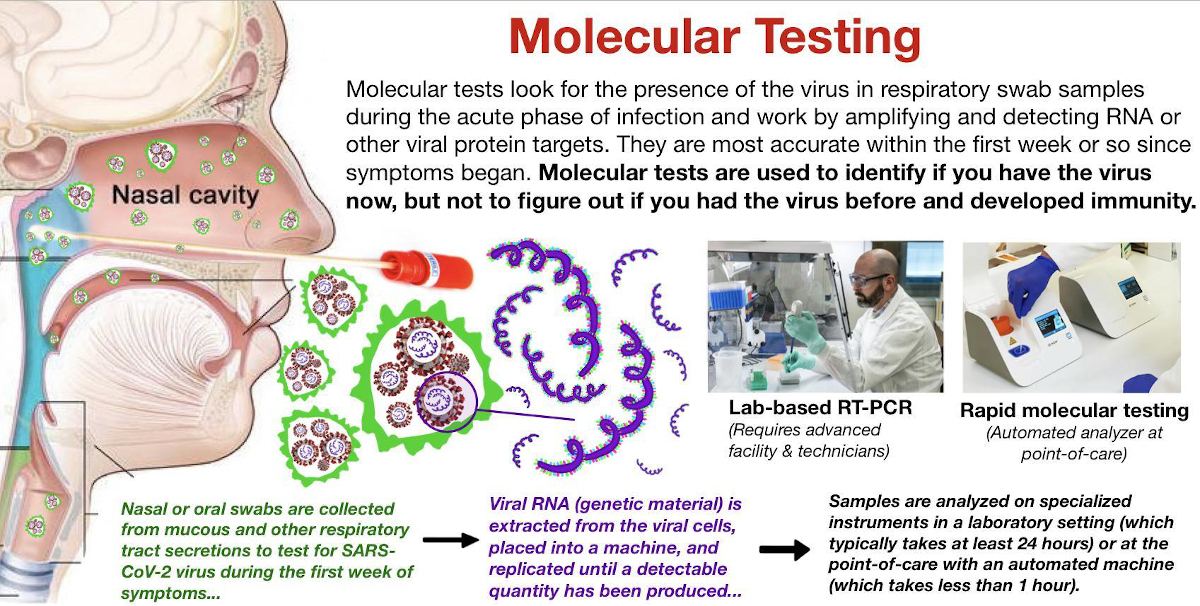
Viral biomarkers (those from the actual virus) that can be detected through testing include specific proteins present on the outside of the virus (“antigensâ€) or viral genetic material (“RNAâ€), and these tests primarily fall into the category of molecular testing.
Most antigen tests are designed to detect the presence of the signature coronavirus spike protein, which covers the outside of the virus and functions like a trojan horse by allowing it to enter into a human cell (“host cellâ€).
Once inside the cell:
- The virus hijacks the cellular machinery of the host and creates millions of copies of itself by forcing the host cell to replicate its genetic material
- The copies eventually burst out of the infected cell and repeat the process throughout the body.
Oral and nasal swab or saliva diagnostic tests are designed to detect these components of the virus.
Rapid Diagnostic Tests (RDT)
Most of the rapid diagnostic tests or RDTs, available for COVID-19 are classified as molecular tests. Molecular testing is advantageous because it can be used to detect the virus earlier in the course of illness before the patient mounted an effective immune response to the virus.
Molecular tests are helpful for identifying and isolating patients before they are able to spread the virus to others and have become an important component of public health strategy.
Several antibody rapid tests exist, but they are of limited utility in most cases because they may be able to tell whether you have been previously exposed to the virus, but they cannot be used to confirm an active infection.
Comparing RDTs to Polymerase Chain Reaction (PCR) Tests
Polymerase chain reaction testing, also known as PCR, works like a copy machine to amplify the concentration of viral biomarkers in a sample. This allows scientists to take a sample with a very small amount of virus and cycle it through the PCR machine multiple times until it has generated high enough levels to be detected.
PCR tests offer the highest degree of accuracy because they can identify trace amounts of viral material in a sample, but they also require time, sophisticated laboratory equipment, and highly trained personnel to process a large batch of samples through multiple PCR cycles. In contrast to PCR tests, RDTs are designed to offer portability, scalability, and speed.
However, these benefits cannot be achieved without sacrificing some degree of precision of PCR tests.
Benefits of RDTs
RDTs can be:
- Simple and visually interpreted without specialized equipment, OR
- More complex and require a portable analyzer to process the sample.
Visual read RDTs:
- Work similarly to a home pregnancy test.
- Can be collected and interpreted almost anywhere, without the use of specialized laboratory equipment.
RDTs with portable analyzers:
- Are more sophisticated than visually read types.
- Often require a proprietary cartridge and specialized reagents, which may be in short supply
- Have a higher cost of equipment, which may in some cases be offset by increased test accuracy.
RDT Logistics
Molecular testing detects COVID-19 in the acute phase (early in the course of infection) and is used to diagnose the virus in symptomatic patients.
- Target: Tests for the presence of the actual SARS-CoV-2 virus in the respiratory tract by amplifying and detecting viral genetic material or spike proteins (antigen testing).
- Sample type: Saliva samples or swabs obtained from the respiratory tract (mouth, nose, throat, etc.)
- Timeframe: Best utilized in the first 1 – 2 weeks of the illness when large quantities of the virus are present in the respiratory tract and can be picked up on swabs or saliva samples.
Equipment required: There are rapid molecular tests available using desktop analyzer machines as well as visual read lateral flow RDTs.
- Rapid molecular test with analyzer: Automated, point-of-care (POC) compact desktop analyzers. Most run only one sample at a time using proprietary instruments and cartridges. Results typically take 10 – 45 minutes.
- Antigen RDTs: RDTs are simple point-of-care tests that can be performed in low-resource settings; most are interpreted visually without specialized equipment but several require proprietary reading devices. Results typically provided in 15 – 30 minutes.
If your organization is interested in rapid COVID-19 Testing, RMI is prepared to assist. RMI can provide all of the necessary solutions to deliver rapid on-site testing services, providing your organization with a truly hands-off experience.